Transcriptome features of striated muscle aging and predictability of protein level changes†
Abstract
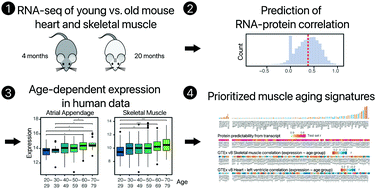
We performed total RNA sequencing and multi-omics analysis comparing skeletal muscle and cardiac muscle in young adult (4 months) vs. early aging (20 months) mice to examine the molecular mechanisms of striated muscle aging. We observed that aging cardiac and skeletal muscles both invoke transcriptomic changes in innate immune system and mitochondria pathways but diverge in extracellular matrix processes. On an individual gene level, we identified 611 age-associated signatures in skeletal and cardiac muscles, including a number of myokine and cardiokine encoding genes. Because RNA and protein levels correlate only partially, we reason that differentially expressed transcripts that accurately reflect their protein counterparts will be more valuable proxies for proteomic changes and by extension physiological states. We applied a computational data analysis workflow to estimate which transcriptomic changes are more likely relevant to protein-level regulation using large proteogenomics data sets. We estimate about 48% of the aging-associated transcripts predict protein levels well (r ≥ 0.5). In parallel, a comparison of the identified aging-regulated genes with public human transcriptomics data showed that only 35–45% of the identified genes show an age-dependent expression in corresponding human tissues. Thus, integrating both RNA–protein correlation and human conservation across data sources, we nominate 134 prioritized aging striated muscle signatures that are predicted to correlate strongly with protein levels and that show age-dependent expression in humans. The results here reveal new details into how aging reshapes gene expression in striated muscles at the transcript and protein levels.


 Please wait while we load your content...
Please wait while we load your content...