Substituent-dependent absorption and fluorescence properties of perylene bisimide radical anions and dianions†‡
Abstract
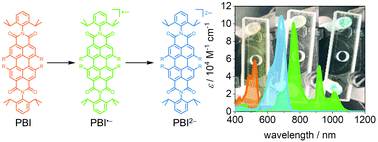
Perylene-3,4:9,10-bis(dicarboximides) (PBIs) rank among the most important functional dyes and organic semiconductors, but only recently have their radical anions and dianions attracted interest for a variety of applications. Here, we systematically elucidate the functional properties (redox, absorption, and emission) of five PBI anions and dianions bearing different bay-substituents attached to the chromophore core. Cyclic voltammetry measurements reveal the influence of the substituents ranging from electron-withdrawing cyano to electron-donating phenoxy groups on the oxidation and reduction potentials that relate to the HOMO and LUMO levels ranging from −7.07 eV to −6.05 eV and −5.01 eV to −4.05 eV, respectively. Spectroelectrochemical studies reveal a significant number of intense absorption bands in the NIR-spectral range (750–1400 nm) for the radical anions, whereas the dianionic species are characterized by similar spectra to those for the neutral dyes, however being bathochromically shifted and with increased molar extinction coefficients of approximately 100 000 M−1 cm−1. The increase of the transition dipole moment is up to 56% and accompanied by an almost cyanine-like red-shifted (by 300 nm) absorption spectrum for the most electron-poor tetracyanotetrachloro PBI. Whilst the outstanding fluorescence properties of the neutral PBIs are lost for the radical anions, an appreciable near-infrared (NIR) fluorescence with a quantum yield of up to 18% is revealed for the dianions by utilizing a custom-built flow-cell spectroelectrochemistry setup. Time-dependent density functional theory calculations help to assign the absorption bands to the respective electronic transitions.
- This article is part of the themed collections: Editor’s Choice collection: Organic Electronics and Special issue in honour of Seth Marder


 Please wait while we load your content...
Please wait while we load your content...