Non-flammable liquid electrolytes for safe batteries
Abstract
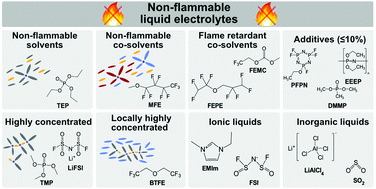
With continual increments in energy density gradually boosting the performance of rechargeable alkali metal ion (e.g. Li+, Na+, K+) batteries, their safe operation is of growing importance and needs to be considered during their development. This is essential, given the high-profile incidents involving battery fires as portrayed by the media. Such hazardous events result from exothermic chemical reactions occurring between the flammable electrolyte and the electrode material under abusive operating conditions. Some classes of non-flammable organic liquid electrolytes have shown potential towards safer batteries with minimal detrimental effect on cycling and, in some cases, even enhanced performance. This article reviews the state-of-the-art in non-flammable liquid electrolytes for Li-, Na- and K-ion batteries. It provides the reader with an overview of carbonate, ether and phosphate-based organic electrolytes, co-solvated electrolytes and electrolytes with flame-retardant additives as well as highly concentrated and locally highly concentrated electrolytes, ionic liquids and inorganic electrolytes. Furthermore, the functionality and purpose of the components present in typical non-flammable mixtures are discussed. Moreover, many non-flammable liquid electrolytes are shown to offer improved cycling stability and rate capability compared to conventional flammable liquid electrolytes.


 Please wait while we load your content...
Please wait while we load your content...