Functionalized highly electron-rich redox-active electropolymerized 3,4-propylenedioxythiophenes as precursors and targets for bioelectronics and supercapacitors†
Abstract
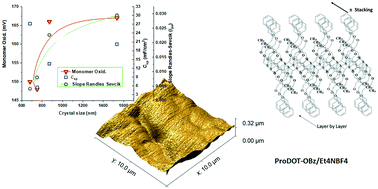
In order to combine capacitive properties with processability, e.g. solubility in organic solvents, poly(3,4-propylenedioxythiophene) derivatives containing different functional groups like oxyphenyl methanol (–OPhCH2OH), oxybenzyl (–OBz), bromide (–Br) and tosyl (–OTs) were synthesized and electropolymerized as thin films from acetonitrile (ACN) using Et4NBF4 as an electrolyte. Multifunctionality in the substitution pattern of the polymer exhibits a similar trend between monomer oxidation potentials and specific capacitance (Csp) vs. crystal size. The presence of π–π stacking interactions in the polymer structures plays an important role in building the crystal structures. The same order of flat band potential and Csp values are observed for –OBz < –Br < –OTs < –OPhCH2OH substitutions. The structures of PProDOT–OBz and PProDOT–OPhCH2OH resemble each other much more than those of PProDOT–Br and PProDOT–OTs. Impedance measurements were conducted at different applied biases in order to define a Mott–Schottky analysis revealing the dependence of the semiconducting properties on the type of substituent present in the PProDOT derivative.


 Please wait while we load your content...
Please wait while we load your content...