Phenyl bioisosteres in medicinal chemistry: discovery of novel γ-secretase modulators as a potential treatment for Alzheimer's disease†
Abstract
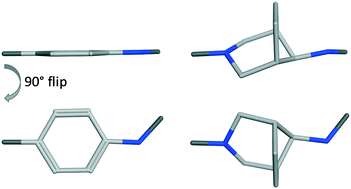
Phenyl rings are one of the most prevalent structural moieties in active pharmaceutical ingredients, even if they often contribute to poor physico-chemical properties. Herein, we propose the use of a bridged piperidine (BP) moiety as a phenyl bioisostere, which could also be seen as a superior phenyl alternative as it led to strongly improved drug like properties, in terms of solubility and lipophilicity. Additionally, this BP moiety compares favorably to the recently reported saturated phenyl bioisosteres. We applied this concept to our γ-secretase modulator (GSM) project for the potential treatment of Alzheimer's disease delivering clinical candidates.
- This article is part of the themed collection: NeuroMedChem

 Please wait while we load your content...
Please wait while we load your content...