Silicon oxycarbonitride ceramic containing nickel nanoparticles: from design to catalytic application†
Abstract
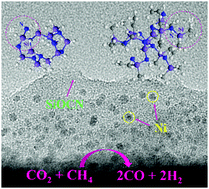
Nickel-containing silicon oxycarbonitride ceramic nanocomposites are synthesized from hydrous nickel acetate and poly(vinyl)silazane (Durazane 1800) or perhydropolysilazane NN120-20 (A) (PHPS). A room temperature chemical reaction results in Ni-containing polysilazane precursors which are transformed into ceramic nanocomposites with nickel nanoparticles (2–4 nm) upon pyrolysis at elevated temperatures (700–1100 °C) under an argon atmosphere. The ceramic nanocomposites derived from the Durazane 1800-Ni precursor by the thermolysis process at 700 and 900 °C manifest a microporous structure with a BET specific surface area of ∼361 and ∼232 m2 g−1, respectively. In contrast, all pyrolyzed samples derived from the PHPS-Ni precursor exhibit a nonporous structure. The Ni/SiOCN ceramic nanocomposites – tested in a plug-flow fixed-bed reactor – display significant catalytic activity in dry methane reforming to syngas. The highest CH4 reaction rate of 0.18 mol min−1 gNi−1 is observed at 800 °C for the sample derived from the PHPS-Ni precursor by pyrolysis at 900 °C. All these make the materials developed in this work, i.e. nickel nanoparticles in situ formed in the SiOCN ceramic matrix, as promising candidates for heterogeneous catalysis.


 Please wait while we load your content...
Please wait while we load your content...