Probing the unpaired Fe spins across the spin crossover of a coordination polymer†
Abstract
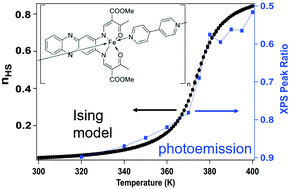
For the spin crossover coordination polymer [Fe(L1)(bipy)]n (where L1 is a N2O22− coordinating Schiff base-like ligand bearing a phenazine fluorophore and bipy = 4,4′-bipyridine), there is compelling additional evidence of a spin state transition. Both Fe 2p X-ray absorption and X-ray core level photoemission spectroscopies confirm that a spin crossover takes place, as observed by magnetometry. Yet the details of the temperature dependent changes of the spin state inferred from both X-ray absorption and X-ray core level photoemission, differ from magnetometry, particularly with regard to the apparent critical transition temperatures and the cooperative nature of the curve progression in general. Comparing the experimental spin crossover data to Ising model simulations, a transition activation energy in the region of 160 to 175 meV is indicated, along with a nonzero exchange J. Overall, the implication is that there may be perturbations to the bistability of spin states, that are measurement dependent or that the surface differs from the bulk with regard to the cooperative effects observed upon spin transition.


 Please wait while we load your content...
Please wait while we load your content...