Microfluidic mass transfer of CO2 at elevated pressures: implications for carbon storage in deep saline aquifers†
Abstract
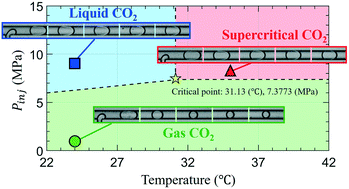
Carbon capture and sequestration (CCS) in a deep saline aquifer is one of the most promising technologies to mitigate anthropologically emitted carbon dioxide. Accurately quantifying the mass transport of CO2 at pore-scales is crucial but challenging for successful CCS deployment. Here, we conduct high-pressure microfluidic experiments, mimicking reservoir conditions up to 9.5 MPa and 35 °C, to elucidate the microfluidic mass transfer process of CO2 at three different states (i.e., gas, liquid, and supercritical phase) into water. We measure the size change of CO2 micro-bubbles/droplets generated using a microfluidic T-junction to estimate the volumetric mass transfer coefficient (kLa), quantifying the rate change of CO2 concentration under the driving force of concentration gradient. The results show that bubbles/droplets under high-pressure conditions reach a steady state faster than low pressure. The measured volumetric mass transfer coefficient increases with the Reynolds number (based on the liquid slug) and is nearly independent of the injection pressure for both the gas and liquid phases. In addition, kLa significantly enlarges with increasing high pressure at the supercritical state. Compared with various chemical engineering applications using millimeter-sized capillaries (with typical kLa measured ranging from ≈0.005 to 0.8 s−1), the microfluidic results show a significant increase in the volumetric mass transfer of CO2 into water by two to three orders of magnitude, O (102–103), with decreasing hydrodynamic diameter (of ≈50 μm).


 Please wait while we load your content...
Please wait while we load your content...