Cardiovascular microphysiological systems (CVMPS) for safety studies – a pharma perspective
Abstract
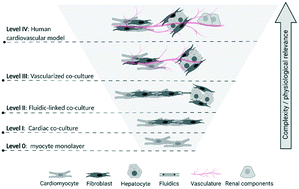
The integrative responses of the cardiovascular (CV) system are essential for maintaining blood flow to provide oxygenation, nutrients, and waste removal for the entire body. Progress has been made in independently developing simple in vitro models of two primary components of the CV system, namely the heart (using induced pluripotent stem-cell derived cardiomyocytes) and the vasculature (using endothelial cells and smooth muscle cells). These two in vitro biomimics are often described as immature and simplistic, and typically lack the structural complexity of native tissues. Despite these limitations, they have proven useful for specific “fit for purpose” applications, including early safety screening. More complex in vitro models offer the tantalizing prospect of greater refinement in risk assessments. To this end, efforts to physically link cardiac and vascular components to mimic a true CV microphysiological system (CVMPS) are ongoing, with the goal of providing a more holistic and integrated CV response model. The challenges of building and implementing CVMPS in future pharmacological safety studies are many, and include a) the need for more complex (and hence mature) cell types and tissues, b) the need for more realistic vasculature (within and across co-modeled tissues), and c) the need to meaningfully couple these two components to allow for integrated CV responses. Initial success will likely come with simple, bioengineered tissue models coupled with fluidics intended to mirror a vascular component. While the development of more complex integrated CVMPS models that are capable of differentiating safe compounds and providing mechanistic evaluations of CV liabilities may be feasible, adoption by pharma will ultimately hinge on model efficiency, experimental reproducibility, and added value above current strategies.
- This article is part of the themed collections: Microphysiological systems in pharmaceutical safety and ADME applications and organ-on-a-chip systems: translating concept into practice


 Please wait while we load your content...
Please wait while we load your content...