High throughput transepithelial electrical resistance (TEER) measurements on perfused membrane-free epithelia†
Abstract
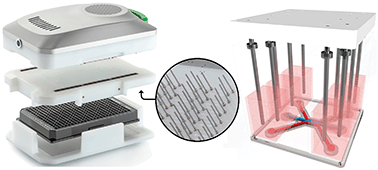
Assessment of epithelial barrier function is critically important for studying healthy and diseased biological models. Here we introduce an instrument that measures transepithelial electrical resistance (TEER) of perfused epithelial tubes in the microfluidic OrganoPlate platform. The tubules are grown in microfluidic channels directly against an extracellular matrix, obviating the need for artificial filter membranes. We present TEER measurements on Caco-2 intestinal and renal proximal tubule epithelium. Forty tubules on one single plate were interrogated in less than a minute. We show that TEER measurement is significantly more sensitive than a fluorescent reporter leakage assay in response to staurosporine. We demonstrate a 40-channel time-lapse data acquisition over a 25 hour time period under flow conditions. We furthermore observed a 50% reduction in Caco-2 TEER values following exposure to a cocktail of inflammatory cytokines. To our best knowledge, this is the first instrument of its kind that allows routine TEER studies in perfused organ-on-a-chip systems without interference by artificial filter membranes. We believe the apparatus will contribute to accelerating routine adoption of perfused organ-on-a-chip systems in academic research and in industrial drug development.


 Please wait while we load your content...
Please wait while we load your content...