XAS study of Sn speciation in toothpaste†
Abstract
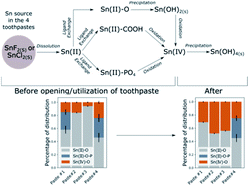
Stannous fluoride (SnF2) is an effective antimicrobial agent and fluoride carrier to dental enamel. However, stannous complexes are known to readily oxidize in presence of O2 and interact with a variety of ligands such as organic and inorganic compounds commonly present in toothpaste. Therefore, the stability of stannous species inside toothpaste remains an open question. Here we probe the speciation of Sn in solutions of known composition and in commercial toothpaste using Sn K-edge X-ray absorption spectroscopy. We show that the oxidation state of Sn and its chemical nature depends on both paste composition and time of opening, with Sn ultimately oxidizing into amorphous Sn(IV) hydroxide, Sn(OH)4. These results highlight the effect of paste composition and utilization on the speciation of active ingredients such as SnF2, and contribute to a rational design of toothpaste formulations that enhances the stability of stannous agents.


 Please wait while we load your content...
Please wait while we load your content...