Reviving electrocatalytic reductive amination: a sustainable route from biogenic levulinic acid to 1,5-dimethyl-2-pyrrolidone†
Abstract
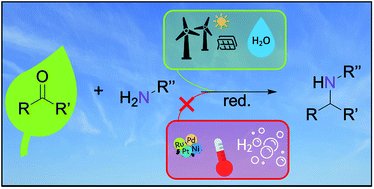
The electrocatalytic reductive amination offers a green pathway to N-containing platform and fine chemicals by using water as a hydrogen source and benign reaction conditions. However, systematic studies about suitable reaction conditions and application to biogenic substrates are rare. Here, we present the electrochemical transformation of levulinic acid to 1,5-dimethyl-2-pyrrolidone. Data from Smirnov et al. for the amination of conventional ketones were validated and extended by systematically investigating the impact of electrode material, substrate concentration, current density, solvent, electrolyte, and pH value. High substrate concentrations in an aqueous electrolyte with a high pH value enable imine formation and copper is identified as the most selective cathode material at current densities lower than 40 mA cm−2. The application of optimized reaction conditions to levulinic acid, followed by a short heating procedure for dehydrative ring closure, led to 1,5-dimethyl-2-pyrrolidone in 78% yield. The systematic approach of this work presents the first example of an electrochemical levulinic acid amination and provides a methodology for the benign synthesis of other N-containing species.


 Please wait while we load your content...
Please wait while we load your content...