Fenton chemistry enables the catalytic oxidative rearrangement of indoles using hydrogen peroxide†
Abstract
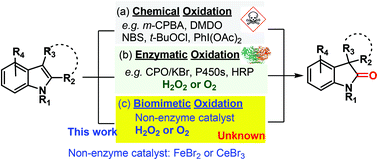
Oxidative rearrangement of indoles is an important transformation to yield 2-oxindoles and spirooxindoles, which are present in many pharmaceutical agents and bioactive natural products. Previous oxidation methods show either broad applicability or greenness but rarely achieve both. Reported is the discovery of Fenton chemistry-enabled green catalytic oxidative rearrangement of indoles, which has wide substrate scope (42 examples) and greenness (water as the only stoichiometric byproduct) at the same time. Detailed mechanistic studies revealed that the Fenton chemistry generated hydroxyl radicals that further oxidize bromide to reactive brominating species (RBS: bromine or hypobromous acid). This in situ generated RBS is the real catalyst for the oxidative rearrangement. Importantly, the RBS is generated under neutral conditions, which addresses a long-lasting problem of many haloperoxidase mimics that require a strong acid for the oxidation of bromide with hydrogen peroxide. It is expected that this new catalytic Fenton-halide system will find wide applications in organic synthesis.
- This article is part of the themed collection: 2021 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...