Regio- and stereoselective electrochemical synthesis of sulfonylated enethers from alkynes and sulfonyl hydrazides†
Abstract
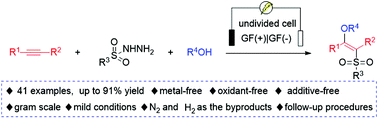
An electrooxidative direct difunctionalization of internal alkynes with sulfonyl hydrazides has been developed for the construction of sulfonated enethers. In this transformation, metal catalysts or stoichiometric amount of oxidants are not required and molecular nitrogen and hydrogen are the sole byproducts, providing a simple and green approach for preparing various sulfonyl tetrasubstituted alkenes. Notably, the protocol could be efficiently scaled up and the follow-up procedures of the corresponding functionalized alkenes demonstrate the practicality of the electrochemical synthesis.


 Please wait while we load your content...
Please wait while we load your content...