A modular, low footprint and scalable flow platform for the expedient α-aminohydroxylation of enolizable ketones†
Abstract
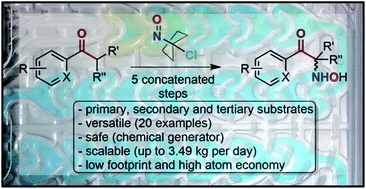
The unique reactivity profile of α-chloronitroso derivatives is expressed to its fullest potential through the development of an integrated, modular and scalable continuous flow process for the electrophilic α-aminohydroxylation of various enolizable ketones. Flow conditions contribute to mitigating the high reactivity and toxicity of α-chloronitroso derivatives and provide an efficient, versatile and safe protocol for the α-aminohydroxylation of ketones with a minimal footprint. Fundamental aspects of the α-aminohydroxylation process were computed by DFT and further supported the experimental observations, hence leading to the unprecedented α-chloronitroso-based α-aminohydroxylation of primary, secondary and tertiary substrates. Recycling of the carbon backbone of the α-chloronitroso derivatives provides a high atom economy for the preparation of value-added molecules. This work showcases α-chloronitroso derivatives as economic and efficient vehicles for transferring electrophilic synthons of hydroxylamine toward nucleophilic enolates. A representative range of precursors and analogs of pharmaceutical active ingredients, including WHO essentials and drugs in shortage (such as epinephrine and ketamine), are prepared within minutes according to a fully concatenated process. The process features sequential modules with distinct unit operations including chemical transformations and multiple in-line extractions. The process relies on an upstream chemical Generator that manages the preparation of α-chloronitroso derivatives and that feeds downstream a series of α-aminohydroxylation modules. The setup is amenable to the addition of libraries of compounds for feeding upstream the process of discovery in medicinal chemistry and is transposable to pilot scale. Several layers of in-line analytical procedures are featured to improve process control and safety.


 Please wait while we load your content...
Please wait while we load your content...