Abstract
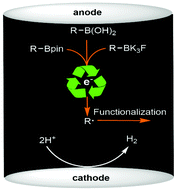
Radical cleavage of C–B bonds to accomplish C–H functionalization is synthetically appealing but practically challenging. We report herein a mild electro-oxidative method for efficient C–H alkylation of quinoxalin-2(1H)-ones by means of radical addition reactions of alkyl boronic acids and esters and alkyl trifluoroborates to afford C–C coupled products.


 Please wait while we load your content...
Please wait while we load your content...