Selective extraction of lithium from a spent lithium iron phosphate battery by mechanochemical solid-phase oxidation†
Abstract
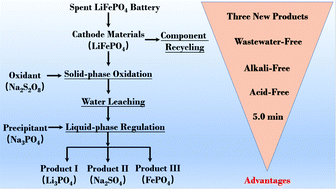
This study proposes a green process for selective and rapid extraction of lithium from the cathode materials of spent lithium iron phosphate (LiFePO4) batteries via mechanochemical solid-phase oxidation. The advantages of the designed process are: (1) acid/base free; (2) extremely short time (5.0 min); (3) wastewater-free discharge; (4) three new chemical products; (5) high economic profit. The results show that by using solid-phase oxidant sodium persulfate (Na2S2O8) as a mechanochemical co-grinding agent and by carrying out the mechanochemical reaction under optimal conditions (rotary speed of 600 rpm, reaction time of 5.0 min, and Na2S2O8 : LiFePO4 mass ratio of 2 : 1), 99.7 wt% of the lithium in LiFePO4 can be selectively released and converted to lithium sulphate (Li2SO4), while the iron phosphate maintains an olivine frame structure. Lithium can then be recovered as a lithium phosphate (Li3PO4) product by integrated water leaching and chemical precipitation. Mechanism analysis shows that mechanochemical-assisted solid-phase oxidation may be a new approach for the selective release of lithium from LiFePO4; lithium behaves differently in this process compared to liquid-phase oxidation. This study provides a new approach to the selective and green recovery of lithium in spent LiFePO4 batteries.


 Please wait while we load your content...
Please wait while we load your content...