A sustainable and scalable multicomponent continuous flow process to access fused imidazoheterocycle pharmacophores†
Abstract
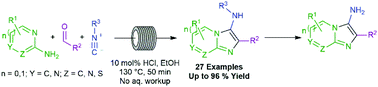
Described herein is a green, continuous flow process for the synthesis of various aminoimidazoheterocycles, through the Gröebke–Blackburn–Bienaymé reaction (GBBR). This multicomponent procedure combines aminoazines, aldehydes and isocyanides to generate a wide variety of medicinally privileged, aminated imidazoheterocycle architectures. This method is performed in ethanol, using only mineral acid rather than the standard metal-based catalysts typical to the field. These sustainability benefits have been demonstrated even on multigram scale, exemplifying the facile scalability of the procedure. The process also boasts shorter reaction times, wider scope robustness, and improved yields compared to the currently available methods, with no requirement for an aqueous work-up procedure, affording resulting scaffolds of notable relevance, to a range of medicinal targets of academic and industrial interest.
- This article is part of the themed collection: 2021 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...