Direct dimethyl carbonate synthesis from CO2 and methanol catalyzed by CeO2 and assisted by 2-cyanopyridine: a cradle-to-gate greenhouse gas emission study†
Abstract
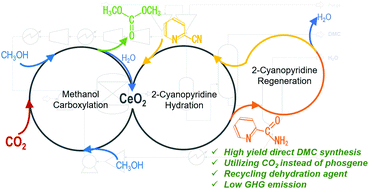
The direct synthesis of dimethyl carbonate (DMC) from CO2 and methanol is an attractive alternative route utilizing CO2 instead of toxic phosgene as a carbonate source. The route is thermodynamically difficult because of the equilibrium limitation of the reaction 2CH3OH + CO2 → (CH3O)2O + H2O. In addition, the azeotrope formed by DMC and methanol makes the separation of DMC from unreacted methanol complex and energy intensive. The use of CeO2 and 2-cyanopyridine as a catalyst and dehydration agent solved both the equilibrium constraint and the separation challenge. In this study, the direct DMC synthesis from CO2 and methanol over CeO2 with 2-cyanopyridine was evaluated in terms of greenhouse gas (GHG) emission with the aid of process simulation. It was validated that the cradle-to-gate greenhouse gas emission attributed to the product of this system (0.39 kg-CO2-eq per kg-DMC) becomes much lower than that in conventional commercialized processes. The heat exchange in the process reduced the emission further to 0.34 kg-CO2-eq per kg-DMC. Among the items associated with emissions, methanol consumption shared the largest part (0.63 kg-CO2-eq per kg-DMC), while the converted CO2 was regarded as an important offset (−0.49 kg-CO2-eq per kg-DMC). It is due to the use of the typical methanol production from natural gas (0.88 kg-CO2-eq per kg-methanol). It suggests that if the methanol production with its associated GHG emission accounting for less than 0.41 or 0.34 kg-CO2-eq per kg-methanol is applicable for with or without heat-exchanging cases, the presented process achieves negative emission. Furthermore, based on the results, the requirements for the practical process implementation are discussed by comparing the lifecycle GHG emission results with other DMC synthesis routes.


 Please wait while we load your content...
Please wait while we load your content...