Understanding plasma–ethanol non-equilibrium electrochemistry during the synthesis of metal oxide quantum dots†
Abstract
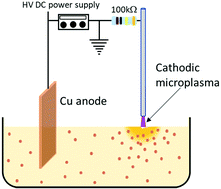
Plasma–liquid interactions are becoming increasingly interesting due to their key features such as non-faradaic, non-equilibrium behaviour as well as electron-driven reactions, therefore with potential strong impact for several promising applications. However, understanding reaction mechanisms initiated at the plasma–liquid interface is complicated by short timescales and spatial non-uniformities. Here we study a plasma–ethanol system that has general relevance to broaden our understanding of plasma interacting at the surface of a liquid. This plasma-electrochemical approach has been successfully used to synthesize a range of metal–oxide nanoparticles and quantum dots (QDs). While nanoparticles and QDs can be an end to this process, they can also be viewed as ‘chemical probes’ that help understanding the underlying and progenitor chemical reactions. We have therefore studied plasma–ethanol interactions during the synthesis of CuO QDs. The colloid was characterised by Fourier transform infrared spectroscopy, ultraviolet-visible spectroscopy, nuclear magnetic resonance spectroscopy and gas chromatography-mass spectrometry. Further, measurements for pH and other trace products were also carried out. The analysis shows the acidolysis of the ethanol electrolyte where hydrogen peroxide was found after the plasma process. A semi-quantification of Cu ions was carried out to confirm the anodic dissolution of the Cu metal foil. Thus, a detailed set of reactions are proposed and has been discussed in detail. Material characterisation relied on transmission electron microscopy and X-ray photoelectron spectroscopy which provided important and complementary information to corroborate chemical reaction paths.
- This article is part of the themed collection: 2021 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...