Integration of aprotic CO2 reduction to oxalate at a Pb catalyst into a GDE flow cell configuration†
Abstract
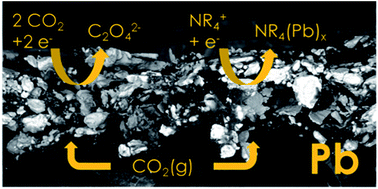
Electrochemical CO2 reduction to oxalic acid in aprotic solvents could be a potential pathway to produce carbon-neutral oxalic acid. One of the challenges in aprotic CO2 reduction are the limited achievable current densities under standard conditions, despite the increased CO2 solubility compared to aqueous applications. The application of aprotic solvents can reduce CO2 rather selectively to oxalate, and faradaic efficiencies (FEs) of up to 80% were achieved in this study with a Pb catalyst in acetonitrile, the FE being mainly dictated by the local CO2 concentration at the electrode. This process was integrated into a flow cell employing a two-layered carbon-free lead (Pb) gas diffusion electrode (GDE) and a sacrificial zinc (Zn) anode. With the application of this GDE the applicable current densities could be improved up to a current density of j = 80 mA cm−2 at a FE(oxalate) = 53%, which is within the range of the highest j reported in the literature. In addition, we provide an explanation for the deactivation mechanism of metal catalysts observed in the aprotic CO2 reduction literature. The deactivation is not related to a mass transport limitation but to cathodic corrosion observed at highly negative potential when employing quaternary ammonium supporting electrolyte cations, promoting catalyst leaching.
- This article is part of the themed collection: Carbon dioxide utilisation


 Please wait while we load your content...
Please wait while we load your content...