Properties and reactivity of sulfidized nanoscale zero-valent iron prepared with different borohydride amounts†
Abstract
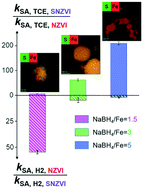
The liquid-phase reduction method with NaBH4 as the reductant is the most widely used method for sulfidized nanoscale zero-valent iron (SNZVI) synthesis. However, it is unclear how the reductant amount (e.g., NaBH4/Fe ratio) affects the physicochemical properties (i.e. Fe0 and S content, surface S speciation, and lattice constant of the Fe BCC structure) and reactivity of SNZVI. Herein, we synthesized SNZVI with different NaBH4/Fe ratios to assess how the NaBH4/Fe ratio affects their properties, and how these properties correlated to the reactivity and selectivity. The S/Fe molar ratio in the particles of SNZVI decreased from 0.15 to 0.02 when the NaBH4/Fe ratio increased from 1.5 to 5, respectively; meanwhile, the yield of SNZVI increased from 20% to 97%. The Fe0 content and the surface S2−/S22− molar ratio ([S2−/S22−]surface) increased with the increase of the NaBH4/Fe ratio, which enhanced the electron transfer of SNZVI. The trichloroethylene reactivity increased ∼8.5-fold when the [S2−/S22−]surface of SNZVI increased from 0.85 to 1.62, while the selectivity decreased from 82.8% to 64.4% accordingly, indicating that [S2−/S22−]surface could be an indicator for predicting the reactivity and selectivity of SNZVI. These results indicate that the NaBH4/Fe ratio is a possible reason for the lab-to-lab variability of SNZVI reactivity. A higher NaBH4/Fe ratio was favorable for the TCE dechlorination reaction while a lower NaBH4/Fe ratio could enhance the electron selectivity.


 Please wait while we load your content...
Please wait while we load your content...