As(iii) adsorption–oxidation behavior and mechanisms on Cr(vi)-incorporated schwertmannite†
Abstract
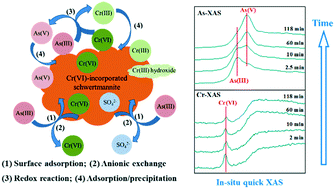
Schwertmannite, chromate (Cr(VI)), and arsenite (As(III)) usually coexist in acid mine drainage (AMD) and surrounding areas, but their interactions and mechanisms are poorly understood. We have determined the behavior and mechanisms of As(III) adsorption–oxidation on Cr(VI)-incorporated schwertmannite (Cr-Sch), with co-adsorption and redox of As(III) and Cr(VI) on pure schwertmannite as a comparison, using batch experiments combined with in situ quick X-ray absorption spectroscopy (Q-XAS). As(III) adsorption isotherms on Cr-Sch can be better described by the Freundlich equation, ascribed to the presence of multiple heterogeneity adsorption sites. With increasing Cr(VI) incorporation, both As(III) adsorption and oxidation increase remarkably. Additionally, with increasing pH from 3 to 7, As(III) adsorption increases, whereas its oxidation increases up to pH 5 and then decreases at higher pH due to the surface passivation by the generated Cr(III) precipitates. Cr(VI) speciation (aqueous vs. structural) seems to have no significant effects on As(III) adsorption–oxidation if structural Cr(VI) is available. The As(III) adsorption–oxidation pathways and mechanisms on Cr-Sch include, firstly, adsorption of As(III) through surface complexation and anionic exchange, followed by a direct redox between As(III) and Cr(VI) on the surface; the newly generated As(V) and Cr(III) then co-adsorb or precipitate on the mineral surface, simultaneously decreasing their toxicity and mobility. These new insights are essential to predict the mobility and availability of As and Cr in schwertmannite-rich natural environments.


 Please wait while we load your content...
Please wait while we load your content...