Atmospheric organic complexation enhanced sulfate formation and iron dissolution on nano α-Fe2O3†
Abstract
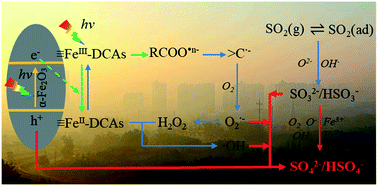
Water-soluble low-molecular-weight dicarboxylic acids (DCAs) and corresponding salts are ubiquitous in the atmosphere. They could efficiently form chelates with iron in atmospheric particles, which could be photoexcited to generate a series of strong oxidants, and have profound impacts on atmospheric chemistry. However, the influences of iron–DCAs complexes on the heterogeneous uptake of atmospheric trace gases are unclear. In this study, the effects of dominant dicarboxylic acid salts (including oxalate, malonate, and succinate) on the heterogeneous SO2 conversion with hematite nanoparticles were systematically investigated using film flow reaction and in situ DRIFTs experiments. It was found that a trace amount of DCA salts could significantly accelerate sulfate formation under solar light illumination. Additionally, similar results were also observed on the Arizona test dust, a proxy of authentic mineral dusts. Moreover, the iron solubility was increased owing to the effects of oxalate complexation, especially under light irradiation. Furthermore, the reaction mechanisms for the enhanced SO2 uptake and iron dissolution in the presence of DCA salts were proposed, and atmospheric environmental implications were discussed.


 Please wait while we load your content...
Please wait while we load your content...