A single-site iron catalyst with preoccupied active centers that achieves selective ammonia electrosynthesis from nitrate†
Abstract
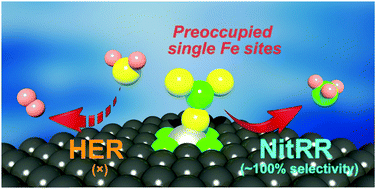
The necessity to pursue sustainable ammonia (NH3) production with economic and environment-friendly technologies is growing with the global development aim of future fertilizer and renewable energy industries. Electrosynthesis of ammonia from nitrate reduction is encouraging for both environmental nitrogen pollution management and artificial nutrient recycling. However, it is fundamentally difficult to regulate the reaction pathways for efficient and selective ammonia production over competing reactions, e.g., the hydrogen evolution reaction, particularly under aqueous conditions. Enlightened by the unique and tunable local electronic structures, an iron-based single-atom catalyst is reported in this contribution. We demonstrate a polymer-hydrogel strategy for preparing nitrogen-coordinated Fe sites with uniform atomic dispersion on carbon. The catalyst exhibits a maximum NH3 yield rate of 2.75 mgNH3 h−1 cm−2 (ca. 30 molNH3 h−1 gFe−1) with nearly 100% faradaic efficiency. Furthermore, the catalytically active individual Fe site in the isolated atom state displays a twelve times higher turnover frequency than that in metallic Fe nanoparticles. Experimental evidence suggests that the single-site iron would experience a nitrate-preoccupied transition center, which prohibits water adsorption as the competitive reaction that generally exists for bulk catalysts. Theoretical insights into the localized structure further assist in better understanding and support of the high selectivity for NH3 achieved by the Fe single-atom catalyst.


 Please wait while we load your content...
Please wait while we load your content...