Electrocatalytic water splitting with unprecedentedly low overpotentials by nickel sulfide nanowires stuffed into carbon nitride scabbards†
Abstract
Electrocatalytic splitting of water to oxygen and hydrogen is one of the most promising approaches for sustainable production of hydrogen as a carbon-neutral fuel. To establish efficient electrocatalytic water splitting, the overall overpotential for this reaction must be minimized via developing efficient catalysts to promote oxygen and hydrogen evolution at the anode and the cathode, respectively. However, the overpotentials 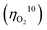 for oxygen evolution are insufficiently low (162–300 mV for a current density of 10 mA cm−2), and the
for oxygen evolution are insufficiently low (162–300 mV for a current density of 10 mA cm−2), and the 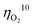 value less than 100 mV still remains untracked. Here, we report the unprecedentedly low
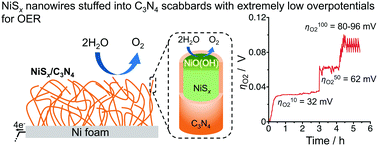
value less than 100 mV still remains untracked. Here, we report the unprecedentedly low 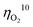 of 32 mV for oxygen evolution attained by the formation of a unique motif of nickel sulfide nanowires stuffed into carbon nitride scabbards (NiSx/C3N4), demonstrating electrocatalytic water splitting at the lowest overall overpotential of 72 mV using the NiSx/C3N4 anode. This motif provides a key to guided thought for the development of efficient catalysts for oxygen evolution.
of 32 mV for oxygen evolution attained by the formation of a unique motif of nickel sulfide nanowires stuffed into carbon nitride scabbards (NiSx/C3N4), demonstrating electrocatalytic water splitting at the lowest overall overpotential of 72 mV using the NiSx/C3N4 anode. This motif provides a key to guided thought for the development of efficient catalysts for oxygen evolution.
- This article is part of the themed collection: SDG12: Sustainable production of energy materials


 Please wait while we load your content...
Please wait while we load your content...