Realizing 6.7 wt% reversible storage of hydrogen at ambient temperature with non-confined ultrafine magnesium hydrides†
Abstract
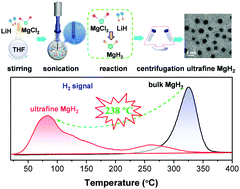
Using light metal hydrides as hydrogen carriers is of particular interest for safe and compact storage of hydrogen. Magnesium hydride (MgH2) has attracted significant attention due to its 7.6 wt% hydrogen content and the natural abundance of Mg. However, bulk MgH2 is stable (ΔHf ∼ 76 kJ mol−1) and releases hydrogen only at impractically high temperatures (>300 °C). Herein, we demonstrate a first attempt to achieve ambient-temperature reversibility of hydrogen storage for MgH2 by fabricating non-confined ultrafine nanoparticles. Taking advantage of the big discrepancies in the solubility of metal hydrides and chlorides in THF, a novel metathesis process of liquid–solid phase driven by ultrasound was proposed. Ultrafine MgH2 nanoparticles predominantly of around 4–5 nm in size were successfully obtained without scaffolds or supports. A reversible hydrogen storage capacity of 6.7 wt% at 30 °C was measured, which has never been achieved before, thanks to thermodynamic destabilization and decreased kinetic barriers. The bare nanoparticles exhibited a stable and rapid hydrogen cycling behaviour in 50 cycles at 150 °C, a remarkable improvement compared with bulk MgH2. Our finding brings MgH2 a step closer to practical applications and the methodology presented here opens new pathways for fabricating sensitive nanoparticles.
- This article is part of the themed collection: Energy Frontiers: Hydrogen


 Please wait while we load your content...
Please wait while we load your content...