Mechanistic insights into the phase transition and metal ex-solution phenomena of Pr0.5Ba0.5Mn0.85Co0.15O3−δ from simple to layered perovskite under reducing conditions and enhanced catalytic activity†
Abstract
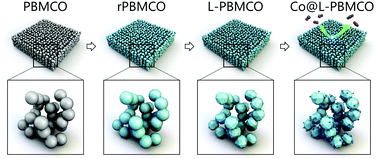
We use density functional theory calculations and in situ X-ray diffraction spectroscopy experiments on Co-doped Pr0.5Ba0.5MnO3−δ (PBMCO) to understand how the phase transition from PBMCO to layered PBMCO occurred. The role of Co dopants for both the phase transition and the ex-solution is also elucidated. It turns out that the selective formation of oxygen vacancies at the Pr layer plays a key role in the phase transition to layered perovskite. The ex-solved Co nanoparticles showed higher catalytic activity than the doped one for CO oxidation. These results can guide the design of highly-active perovskite-based redox catalysts.


 Please wait while we load your content...
Please wait while we load your content...