Self-discharge of magnesium–sulfur batteries leads to active material loss and poor shelf life†
Abstract
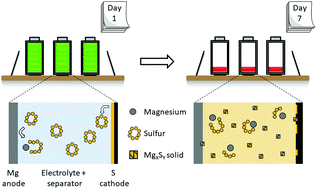
Due to its high theoretical energy density and relative abundancy of active materials, the magnesium–sulfur battery has attracted research attention in recent years. A closely related system, the lithium-sulfur battery, can suffer from serious self-discharge behavior. Until now, the self-discharge of Mg–S has been rarely addressed. Herein, we demonstrate for a wide variety of Mg–S electrolytes and conditions that Mg–S batteries also suffer from serious self-discharge. For a common Mg–S electrolyte, we identify a multi-step self-discharge pathway. Covalent S8 diffuses to the metal Mg anode and is converted to ionic Mg polysulfide in a non-faradaic reaction. Mg polysulfides in solution are found to be meta-stable, continuing to react and precipitate as solid magnesium polysulfide species during both storage and active use. Mg–S electrolytes from the early, middle, and state-of-the-art stages of the Mg–S literature are all found to enable the self-discharge. The self-discharge behavior is found to decrease first cycle discharge capacity by at least 32%, and in some cases up to 96%, indicating this is a phenomenon of the Mg–S chemistry that deserves focused attention.


 Please wait while we load your content...
Please wait while we load your content...