Formation of Bi2Ir nanoparticles in a microwave-assisted polyol process revealing the suboxide Bi4Ir2O†
Abstract
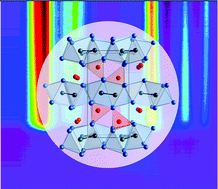
Intermetallic phases are usually obtained by crystallization from the melt. However, phases containing elements with widely different melting and boiling points, as well as nanoparticles, which provide a high specific surface area, are hardly accessible via such a high-temperature process. The polyol process is one option to circumvent these obstacles by using a solution-based approach at moderate temperatures. In this study, the formation of Bi2Ir nanoparticles in a microwave-assisted polyol process was investigated. Solutions were analyzed using UV–Vis spectroscopy and the reaction was tracked with synchrotron-based in situ powder X-ray diffraction (PXRD). The products were characterized by PXRD and high-resolution transmission electron microscopy. Starting from Bi(NO3)3 and Ir(OAc)3, the new suboxide Bi4Ir2O forms as an intermediate phase at about 160 °C. Its structure was determined by a combination of PXRD and quantum-chemical calculations. Bi4Ir2O decomposes in vacuum at about 250 °C and is reduced to Bi2Ir by hydrogen at 150 °C. At about 240 °C, the polyol process leads to the immediate reduction of the two metal-containing precursors and crystallization of Bi2Ir nanoparticles.


 Please wait while we load your content...
Please wait while we load your content...