Reduced quenching effect of pyridine ligands in highly luminescent Ln(iii) complexes: the role of tertiary amide linkers†
Abstract
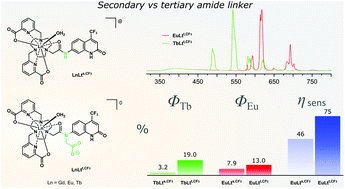
Luminescent Eu(III) and Tb(III) complexes were synthesised from octadentate ligands carrying various carbostyril sensitizing antennae and two bidentate picolinate donors. Antennae were connected to the metal binding site via tertiary amide linkers. Antennae and donors were assembled on a 1,4,7-triazacyclononane (tacn) platform. Solution- and solid-state structures were comparable to those of previously reported complexes with tacn architectures, with nine-coordinate distorted tricapped trigonal prismatic Ln(III) centres, and distinct from those based on 1,4,7,10-tetraazacyclododecane (cyclen) macrocycles. In contrast, the photophysical properties of these tertiary amide tacn-based complexes were more comparable to those of previously reported systems with cyclen ligands, showing efficient Eu(III) and Tb(III) luminescence. This represents an improvement over secondary amide-linked analogues, and is due to a greatly increased sensitization efficiency in the tertiary amide-linked complexes. Tertiary amide-linked Eu(III) and Tb(III) emitters were more photostable than their secondary amide-linked analogues due to the suppression of photoinduced electron transfer and back energy transfer.


 Please wait while we load your content...
Please wait while we load your content...