Synthesis and structure of a phosphinoboronic ester in a fused bicyclic framework†
Abstract
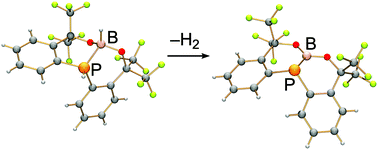
The first phosphinoboronic ester bearing a fused bicyclic framework was synthesised by either deprotonation and hydride abstraction or Rh-catalysed dehydrogenation of a hydrophosphineboronic ester. The phosphinoboronic ester reacted as a Lewis acid with KF/18-crown-6, pyridine and DMAP to give the corresponding adducts. Furthermore, its crystal structure shows a remarkably short P–B bond in comparison with other P–B bonded derivatives in spite of the trigonal pyramidal geometry of the phosphorus. Consistent with the phosphorus pyramidality, the π-type donor–acceptor interaction of the P–B bond is small as revealed by the DFT calculations. The P–B bond shared within the fused six-membered rings has to shorten because of the geometrical requirement and high s-character of the boron.


 Please wait while we load your content...
Please wait while we load your content...