A ruthenium cis-dihydride with 2-phosphinophosphinine ligands catalyses the acceptorless dehydrogenation of benzyl alcohol†‡
Abstract
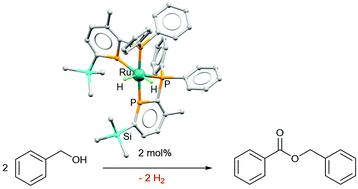
The first ruthenium dihydride complex featuring a phosphinine ligand cis-[Ru(H)2(2-PPh2-3-Me-6-SiMe3-PC5H2)2] was synthesised exclusively as the cis-isomer. When formed in situ from the reaction of cis-[Ru(Cl)2(2-PPh2-3-Me-6-SiMe3-PC5H2)2] with two equivalents of Na[BHEt3], as demonstrated by 31P and 1H NMR spectroscopy, the catalysed acceptorless dehydrogenation of benzyl alcohol was observed leading to benzyl benzoate in up to 70% yield.


 Please wait while we load your content...
Please wait while we load your content...