Energetic isomers of bridged oxadiazole nitramines: the effect of asymmetric heterocyclics on stability and energetic properties†
Abstract
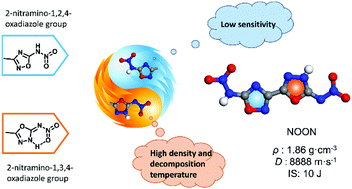
Energetic isomers often exhibit different properties. To understand the effect of arrangement and connection of isomers on energetic properties and sensitivity, in this study, we designed and synthesized a series of oxadiazole nitramine compounds including N-(5-(5-(nitramino)-1,3,4-oxadiazol-2-yl)-1,2,4-oxadiazol-3-yl)nitramide (NOON) and its ionic derivatives. NOON exhibits comparable performance (D = 8888 m s−1, P = 34.1 GPa) to highly explosive RDX. A comparative study of detonation properties, sensitivity, and thermal stability of the three oxadiazole nitramine isomers (NOON, ICM-101, and DNBO) is carried out. The results show that due to the proton transformation, strong intramolecular hydrogen bonding interaction, and formation of six-membered ring conformation, the 2-nitramino-1,3,4-oxadiazole building block exhibits better detonation properties and higher thermal stability than its isomer 2-nitramino-1,2,4-oxadiazole.


 Please wait while we load your content...
Please wait while we load your content...