Metal triflate formation of C12–C22 phenolic compounds by the simultaneous C–O breaking and C–C coupling of benzyl phenyl ether†
Abstract
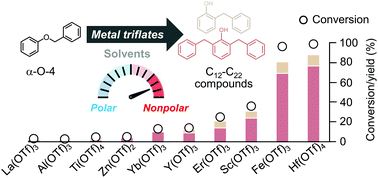
Catalytic pathways to produce high carbon number compounds from benzyl phenyl ether require multiple steps to break the aryl etheric carbon–oxygen bonds; these steps are followed by energy-intensive processes to remove oxygen atoms and/or carbon–carbon coupling. Here, we show an upgrading strategy to transform benzyl phenyl ether into large phenolic (C12–C22) compounds by a one-step C–O breaking and C–C coupling catalyzed by metal triflates under a mild condition (100 °C and 1 bar). Hafnium triflate was the most selective for the desired products. In addition, we measured the effect of solvent polarity on the catalytic performance. Solvents with a polarity index of less than 3.4 promoted the catalytic activity and selectivity to C12–C22 phenolic products. These C12–C22 phenolic compounds have potential applications for phenol–formaldehyde polymers, diesel/jet fuels, and liquid organic hydrogen carriers.


 Please wait while we load your content...
Please wait while we load your content...