Heavy-element–ligand covalence: ligand noninnocence in molybdenum and tungsten Viking-helmet Corroles†
Abstract
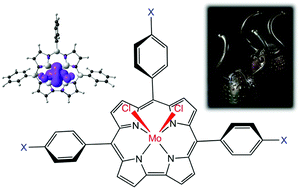
Extensive DFT calculations with several exchange–correlation functionals indicate that molybdenum-dichlorido Viking helmet corroles are noninnocent with significant MoIV-corrole˙2− character. The effect is mediated by a Mo(4d)-corrole(π) orbital interaction similar to that postulated for MnCl, FeCl and FeNO corroles. The effect also appears to operate in tungsten-dichlorido corroles but is weaker relative to that for Mo. In contrast, MoO triarylcorroles do not exhibit a significant degree of corrole radical character. Furthermore, the Soret absorption maxima of a series of MoCl2 tris(para-X-phenyl)corrole derivatives were found to redshift dramatically with increasing electron-donating character of the para substituent X, essentially clinching the case for a noninnocent macrocycle in MoCl2 corroles.


 Please wait while we load your content...
Please wait while we load your content...