Diverse reactions of a fluorostannylenoid towards ethynes†
Abstract
Fluoro(dialkyl)stannylenoid 2 exhibits unique reactivity towards ethynes with acetylenic hydrogen and those with trimethylsilyl groups, though the corresponding free dialkylstannylene 1 is inactive against those ethynes. Stannylenoid 2 reacts smoothly with gaseous ethyne and phenylethyne at room temperature, giving the corresponding diethynylstannanes, di(phenylethynyl)stannane 3 and diethynylstannane 6, respectively, in good yields with the concomitant evolution of H2. Trimethylsilyl-substituted ethynes such as 1-trimethylsilyl-(2-phenyl)ethyne and 1,2-bis(trimethylsilyl)ethyne react similarly to give 3 and bis(trimethylsilylethynyl)stannane 8, respectively. Rather unexpectedly, the reaction of 2 with (trimethylsilyl)ethyne affords 1,2-bis(ethenylstannyl)ethyne 7 in a good yield. The reactions of 2 with methyl and ethyl propynoates give the same products 4 and 5 as those obtained during the reaction of dialkylstannylene 1 without CsF. Pathways involving the nucleophilic attack of cesium acetylide to an ethyne-complexed stannylene were proposed, while the detailed mechanisms remain unknown. The structure of 7 was studied by single crystal X-ray diffraction analysis.
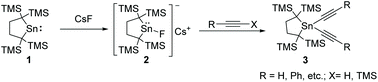
- This article is part of the themed collection: Tin: Modern chemistry of an element from antiquity


 Please wait while we load your content...
Please wait while we load your content...