Mechanistic insights into the α-branched amine formation with pivalic acid assisted C–H bond activation catalysed by Cp*Rh complexes†
Abstract
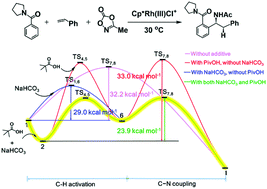
Density functional theory computations revealed a pivalic acid assisted C–H bond activation mechanism for rhodium catalyzed formation of α-branched amines with C–C and C–N bond couplings. The reaction energies of the [Cp*RhCl2]2 dimer and silver cations indicate that the Cp*RhCl+ cation is the active catalyst. The essential role of pivalic acid is a co-catalyst for the activation of the ortho-C(sp2)–H bond in phenyl(pyrrolidin-1-yl)methanone, while the reaction of NaHCO3 and HCl reduces the overall barrier of the catalytic cycle. In the presence of both pivalic acid and NaHCO3 in the reaction, the C(sp2)–H bond is activated through a concerted metallation deprotonation process, and the C–C bond coupling is the rate-determining step with a total free energy barrier of 23.9 kcal mol−1. Without pivalic acid and NaHCO3, the C(sp2)–H bond can only be activated through a σ-bond metathesis process and the free energy barrier increases to 32.2 kcal mol−1. We also investigated the mechanisms of a side reaction for β-branched amine formation and the reaction without styrene and found that their free energy barriers are 33.4 and 30.5 kcal mol−1, respectively.


 Please wait while we load your content...
Please wait while we load your content...