Isonitrile ruthenium and iron PNP complexes: synthesis, characterization and catalytic assessment for base-free dehydrogenative coupling of alcohols†
Abstract
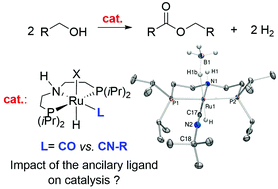
Neutral and ionic ruthenium and iron aliphatic PNHP-type pincer complexes (PNHP = NH(CH2CH2PiPr2)2) bearing benzyl, n-butyl or tert-butyl isocyanide ancillary ligands have been prepared and characterized. Reaction of [RuCl2(PNHP)]2 with one equivalent CN-R per ruthenium center affords complexes [RuCl2(PNHP)(CNR)] (R = benzyl, 1a, R = n-butyl, 1b, R = t-butyl, 1c), with cationic [RuCl(PNHP)(CNR)2]Cl 2a–c as side-products. Dichloride species 1a–c react with excess NaBH4 to afford [RuH(PNHP)(BH4)(CN-R)] 3a–c, analogues to benchmark Takasago catalyst [RuH(PNHP)(BH4)(CO)]. Reaction of 1a–c with a single equivalent of NaBH4 results in formation of [RuHCl(PNHP) (CN-R)] (4a–c), from which 3a–c can be prepared upon reaction with excess NaBH4. Use of one equivalent of NaHBEt3 with 4a and 4c affords bishydrides [Ru(H)2(PNHP)(CN-R)] 5a and 5c. Deprotonation of 4c by KOtBu generates amido derivative [RuH(PNP)(CN-t-Bu)] (6, PNP = −N(CH2CH2PiPr2)2), unstable in solution. Addition of excess benzylisonitrile to 4a provides cationic hydride [RuH(PNHP) (CN-CH2Ph)2]Cl (7). Concerning iron chemistry, [Fe(PNHP)Br2] reacts with one equivalent of benzylisonitrile to afford [FeBr(PNHP)(CNCH2Ph)2]Br (8). The outer-sphere bromide anion can be exchanged by salt metathesis with NaBPh4 to generate [FeBr(PNHP) (CNCH2Ph)2](BPh4) (9). Cationic hydride species [FeH(PNHP) (CN-t-Bu)2](BH4) (10) is prepared from consecutive addition of excess CN-t-Bu and NaBH4 on [Fe(PNPH)Br2]. Ruthenium complexes 3a–c are active in acceptorless alcohol dehydrogenative coupling into ester under base-free conditions. From kinetic follow-up, the trend in initial activity is 3a ≈ 3b > [RuH(PNHP)(BH4)(CO)] ≫ 3c; for robustness, [RuH(BH4)(CO)(PNHP)] > 3a > 3b ≫ 3c. Hypotheses are given to account for the observed deactivation. Complexes 3b, 3c, 4a, 4c, 5c, 7, cis-8 and 9 were characterized by X-ray crystallography.


 Please wait while we load your content...
Please wait while we load your content...