Steric and electronic effects of ligand substitution on redox-active Fe4S4-based coordination polymers†
Abstract
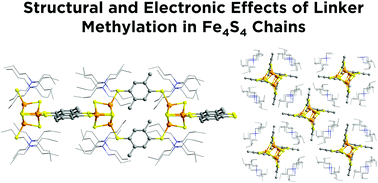
One of the notable advantages of molecular materials is the ability to precisely tune structure, properties, and function via molecular substitutions. While many studies have demonstrated this principle with classic carboxylate-based coordination polymers, there are comparatively fewer examples where systematic changes to sulfur-based coordination polymers have been investigated. Here we present such a study on 1D coordination chains of redox-active Fe4S4 clusters linked by methylated 1,4-benzene-dithiolates. A series of new Fe4S4-based coordination polymers were synthesized with either 2,5-dimethyl-1,4-benzenedithiol (DMBDT) or 2,3,5,6-tetramethyl-1,4-benzenedithiol (TMBDT). The structures of these compounds have been characterized based on synchrotron X-ray powder diffraction while their chemical and physical properties have been characterized by techniques including X-ray photoelectron spectroscopy, cyclic voltammetry and UV–visible spectroscopy. Methylation results in the general trend of increasing electron-richness in the series, but the tetramethyl version exhibits unexpected properties arising from steric constraints. All these results highlight how substitutions on organic linkers can modulate electronic factors to fine-tune the electronic structures of metal–organic materials.


 Please wait while we load your content...
Please wait while we load your content...