Homoleptic quasilinear metal(i/ii) silylamides of Cr–Co with phenyl and allyl functions – impact of the oxidation state on secondary ligand interactions†
Abstract
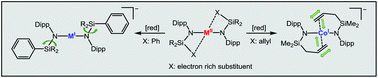
Herein we describe the synthesis and characterization of a variety of new quasilinear metal(I/II) silylamides of the type [M(N(Dipp)SiR3)2]0,− (M = Cr–Co) with different silyl substituents (SiR3 = SiPh3−nMen (n = 1–3), SiMe2(allyl)). By comparison of the solid state structures we show that in the case of phenyl substituents secondary metal–ligand interactions are suppressed upon reduction of the metal. Introduction of an allyl substituted silylamide gives divalent complexes with additional metal–π-alkene interactions with only weak activation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond but substantial bending of the principal N–M–N axis. 1e−-reduction makes cobalt a more strongly bound alkene substituent, whereas for chromium, reduction and intermolecular dimerisation of the allyl unit are observed. It thus indicates that the general view of low-coordinate 3d-metal ions as electron deficient seems not to apply to anionic metal(I) complexes. Additionally, the obtained cobalt(I) complexes are reacted with an aryl azide giving trigonal imido metal complexes. These can be regarded as rare examples of high-spin imido cobalt compounds from their structural and solution magnetic features.
C bond but substantial bending of the principal N–M–N axis. 1e−-reduction makes cobalt a more strongly bound alkene substituent, whereas for chromium, reduction and intermolecular dimerisation of the allyl unit are observed. It thus indicates that the general view of low-coordinate 3d-metal ions as electron deficient seems not to apply to anionic metal(I) complexes. Additionally, the obtained cobalt(I) complexes are reacted with an aryl azide giving trigonal imido metal complexes. These can be regarded as rare examples of high-spin imido cobalt compounds from their structural and solution magnetic features.


 Please wait while we load your content...
Please wait while we load your content...