Hydrogen peroxide production from oxygen and formic acid by homogeneous Ir–Ni catalyst†
Abstract
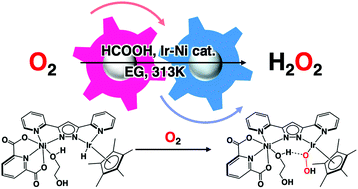
Hydrogen peroxide was directly produced from oxygen and formic acid, catalysed by a hetero-dinuclear Ir–Ni complex with two adjacent sites, at ambient temperature. Synergistic catalysis derived from the hetero-dinuclear Ir and Ni centres was demonstrated by comparing its activity to those of the component mononuclear Ir and Ni complexes. A reaction intermediate of Ir-hydrido was detected by UV-vis, ESI-TOF-MS, and 1H NMR spectroscopies. It was revealed that the Ir moiety serves as an active species of Ir-hydrido, reacting with oxygen to afford an Ir-hydroperoxide species through O2 insertion, which is the rate-determining step for H2O2 production. Meanwhile, the Ni moiety promotes H2O2 formation by activating solvents as proton sources. We also found that H2O2 production is strongly affected by the solvent dielectric constants (DE); the highest H2O2 concentration was obtained in ethylene glycol with a moderate DE. The catalytic mechanism of H2O2 production by the Ir–Ni complex was discussed, based on kinetic analysis, isotope labelling experiments, and theoretical DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...