B–H and O–H bonds activation via a single electron transfer of frustrated radical pairs†
Abstract
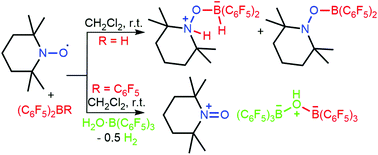
The rare examples of B–H bond activation in a frustrated radical pair regime have been observed by treatment of TEMPO radicals with Piers’ borane HB(C6F5)2 or bis-borane, respectively. The resulting concomitant formation of zwitterionic products and geminal N/B frustrated Lewis pairs implied a one electron process. In addition, the reaction of a TEMPO/B(C6F5)3 pair with H2O·B(C6F5)3 was assumed to involve one-electron reduction of water. Our results provide insights into chemical bond (e.g. B–H and O–H) activation via a single electron transfer.


 Please wait while we load your content...
Please wait while we load your content...