Single-crystal-to-single-crystal transformations among three Mn-MOFs containing different water molecules induced by reaction time: crystal structures and proton conductivities†
Abstract
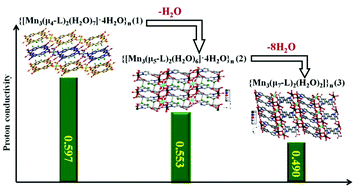
Three Mn-MOFs {[Mn3(μ4-L)2(H2O)7]·4H2O}n (1), {[Mn3(μ5-L)2(H2O)6]·4H2O}n (2) and {[Mn3(μ7-L)2(H2O)2]}n (3) (H3L = 5-(6-carboxypyridin-3-yl)isophthalic acid) were obtained under different reaction times and temperatures. Interestingly, induced by reaction time, compound 1 can lose one water molecule and SC–SC transform into compound 2. Similarly, compound 2 can also SC–SC transform into 3. Studies on two SC–SC transformation processes were carried out and the transformation mechanisms were deduced, which were verified by TG analyses. Different numbers of water molecules in the three compounds resulted in different coordination environments of the metal cation, coordination modes of the L3− ligand, continuities of hydrogen bonds, dimensions of framework and porosities. The AC impendence spectra studies revealed that compounds 1–3 can enhance the proton conductivities of the Nafion composite membrane to about 47.77%, 36.88% and 21.28%, respectively. It is speculated that the highest proton conductivity of compound 1 may be due to its continuous hydrogen bond chain and highest water uptake, which were mainly decided by the number of water molecules.


 Please wait while we load your content...
Please wait while we load your content...