Synthesis, structures and catalytic activity of some BINOL based boronates and boronium salts†
Abstract
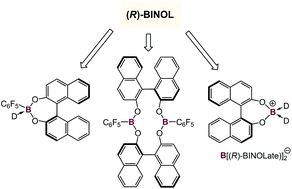
The BINOL supported chiral boronate ester [C10H12O2BC6F5(THF)] [(R)-1], [C10H12O2BC6F5(O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PEt3)] [(R)-3] and [C10H12O2BC6F5]2 [(R,R)-2] as well as the chiral boronium salts [C10H12O2B(O
PEt3)] [(R)-3] and [C10H12O2BC6F5]2 [(R,R)-2] as well as the chiral boronium salts [C10H12O2B(O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PEt3)2]+[B(O2C10H12)2]−, [(R)-6] and [C10H12O2B(O
PEt3)2]+[B(O2C10H12)2]−, [(R)-6] and [C10H12O2B(O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) SMe2)2]+[B(O2C10H12)2]− [(R)-7] have been synthesized, characterized by NMR spectroscopy, and the solid state structures of [(R)-1], [(R,R)-2] and [(R)-3] determined. Chiral ester [(R)-1] was found to be a potent Lewis acid, similar to B(C6F5)3, and capable of rapidly catalyzing the annulation of (R)-, (S)- and rac-styrene oxide with nitrone PhCH
SMe2)2]+[B(O2C10H12)2]− [(R)-7] have been synthesized, characterized by NMR spectroscopy, and the solid state structures of [(R)-1], [(R,R)-2] and [(R)-3] determined. Chiral ester [(R)-1] was found to be a potent Lewis acid, similar to B(C6F5)3, and capable of rapidly catalyzing the annulation of (R)-, (S)- and rac-styrene oxide with nitrone PhCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(O)Me to trans-2-methyl-3,6-diphenyl-1,4,2-dioxazine (trans-11) with high regio- and diastereoslectivities.
N(O)Me to trans-2-methyl-3,6-diphenyl-1,4,2-dioxazine (trans-11) with high regio- and diastereoslectivities.


 Please wait while we load your content...
Please wait while we load your content...