Mixed-valence di-ruthenium(ii,iii) complexes of redox non-innocent N-aryl-o-phenylenediamine derivatives†
Abstract
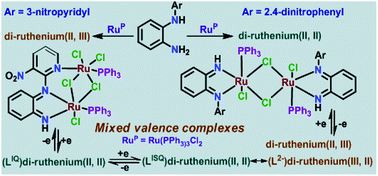
A mononuclear ruthenium(II), [(L1IQ)Ru2+(PPh3)2Cl2]·CHCl3 (1·CHCl3), a di-ruthenium(II,II), [(L2IQ)2Ru24+Cl4(PPh3)2] (2) and a mixed-valence di-ruthenium(II,III), [(L3IQ)Ru25+Cl5(PPh3)2]·MeOH (3·MeOH) complex, where L1IQ, L2IQ and L3IQ are, respectively, o-diiminobenzoquinone forms of redox non-innocent N-(5-nitropyridyl)-o-phenylenediamine (L1H2), N-(2,4-dinitrophenyl)-o-phenylenediamine (L2H2) and N-(3-nitropyridyl)-o-phenylenediamine (L3H2) derivatives, were successfully isolated. The molecular and electronic structures of 1·CHCl3, 2 and 3·MeOH were confirmed by single-crystal X-ray crystallography, EPR, UV-Vis-NIR spectroscopies and density functional theory (DFT) calculations. Both 1·CHCl3 and 2 exhibited reversible anodic waves due to the Ru(III)/Ru(II) redox couple, while the cyclic voltammogram of 3·MeOH displayed two successive cathodic waves due to ruthenium(III)/ruthenium(II) and (L3IQ/L3ISQ) redox couples. EPR spectroscopy and DFT calculations inferred that 1+ is a ruthenium(III) complex of L1IQ, [(L1IQ)Ru3+(PPh3)2Cl2]+, 2+ is a mixed-valence di-ruthenium(II,III) complex of L2IQ, [(L2IQ)2Ru25+Cl4(PPh3)2]+ and 3− is a di-ruthenium(II,II) complex, [(L3IQ)Ru24+Cl5(PPh3)2]−, while 32− is a di-ruthenium(II,II) complex of L3ISQ, [(L3ISQ)Ru24+Cl5(PPh3)2]2−, where L3ISQ is the o-diiminobenzosemiquinonate anion radical form of the L3H2 with a significant contribution of the mixed-valence di-ruthenium(III,II) form, [(L3AM)Ru25+Cl5(PPh3)2]2− (L3AM is the di-amido form of the L3H2 ligand). The spin density obtained from the Mulliken spin population analyses of the broken-symmetry (BS) DFT calculations was localized on Ru(2) in 3, while it was delocalized over both Ru(1) and the o-phenylenediamine fragment in 32−. The inter-valence charge-transfer (IVCT) transitions of 2+ and 3 were relatively weaker and occurred at 1680 and 1685 nm, respectively, while 32− exhibited a broader NIR absorption band at 1500–2000 nm. The calculated electronic-coupling parameters (Hab) using the Mulliken–Hush equation for 3 and 2+ ion, respectively, were 62 and 103 cm−1, defining these as Robin–Day class II mixed-valence systems.


 Please wait while we load your content...
Please wait while we load your content...