A novel approach to characterization of a relatively unstable intercalation compound under ambient conditions: revisiting a kaolinite-acetone intercalation compound†
Abstract
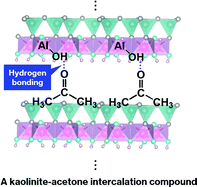
Characteristics of a kaolinite-acetone intercalation compound prepared using a kaolinite N-methylformamide intercalation compound (Kaol-NMF) as an intermediate were obtained by a set of techniques with attention to suppressing evaporation and deintercalation of acetone. X-ray diffraction (XRD) with spectroscopic analyses, Fourier-transform infrared spectroscopy (FTIR) accompanied by solid-state 13C and 29Si nuclear magnetic resonance (NMR) spectroscopy with cross polarization (CP) and magic angle spinning (MAS) enable us to demonstrate full replacement of a pre-intercalated NMF monolayer with an acetone monolayer between the layers of kaolinite with an increase in the basal spacing from 1.08 nm (Kaol-NMF) to 1.12 nm. In addition, the appearance of an additional OH stretching band at 3630 cm−1 and the shift of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching band to a lower wavenumber, from 1714 to 1701 cm−1, in the FTIR spectrum, along with a downfield shift of the signal due to C
O stretching band to a lower wavenumber, from 1714 to 1701 cm−1, in the FTIR spectrum, along with a downfield shift of the signal due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups from 209 ppm, where a singlet was observed in the liquid-state 13C NMR spectrum of acetone in CDCl3, to 219 ppm in the 13C CP/MAS NMR spectrum, indicate hydrogen bond formation between interlayer hydroxyl groups of kaolinite and C
O groups from 209 ppm, where a singlet was observed in the liquid-state 13C NMR spectrum of acetone in CDCl3, to 219 ppm in the 13C CP/MAS NMR spectrum, indicate hydrogen bond formation between interlayer hydroxyl groups of kaolinite and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of the intercalated acetone molecules. These careful characterization studies provide information on an interaction between kaolinite and acetone under ambient conditions.
O groups of the intercalated acetone molecules. These careful characterization studies provide information on an interaction between kaolinite and acetone under ambient conditions.


 Please wait while we load your content...
Please wait while we load your content...