Self-supported wire-in-plate NiFeS/CoS nanohybrids with a hierarchical structure for efficient overall water splitting†
Abstract
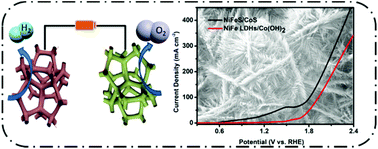
Highly efficient low-cost electrocatalysts play a key role in overall water splitting to generate hydrogen and oxygen. Herein, a self-supported hierarchical NiFeS/CoS nanosheet/nanowire bifunctional electrocatalyst for overall water splitting supported on nickel foam is synthesized by the combined process of hydrothermal and sulfurization methods. The specific wire-in-plate micromorphology of the catalyst provides the advantages of high contact area for electrolyte penetration, extensive active surface area and plentiful accessible active sites. Moreover, the quaternary catalyst in situ grown on the substrate guarantees mechanical stability. Reasonably, the as-obtained NiFeS/CoS catalyst with a unique wire-in-plate nanostructure shows good electrocatalytic performance toward the OER, HER and efficient overall water splitting. The NiFeS/CoS catalyst delivers 50 and 150 mA cm−2 at ultralow overpotentials of 170 and 150 mV toward the OER and HER, respectively. When simultaneously used as the electrocatalyst at both the cathode and the anode of an alkaline electrolyzer, the NiFeS/CoS electrocatalyst requires a cell voltage of 1.81 V at a water-splitting current density of 100 mA cm−2 for overall water splitting. This investigation provides an effective strategy to design hierarchically multidimensional nanohybrids for bifunctional electrocatalysts by combining nanowires with nanoplates.


 Please wait while we load your content...
Please wait while we load your content...