Borane-catalysed dinitrogen borylation by 1,3-B–H bond addition†
Abstract
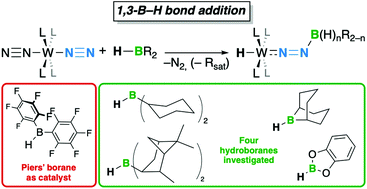
The borylation of ligated dinitrogen by 1,3-B–H bond addition over a W–N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N unit using various hydroboranes has been examined. In a previous study, we have shown that Piers’ borane (1) reacted with the tungsten dinitrogen complex 2 to afford a boryldiazenido–hydrido–tungsten species. The ease and mildness of this reaction have encouraged us to extend its scope, with the working hypothesis that 1 could potentially catalyse the 1,3-B–H bond addition of other hydroboranes. Under productive reaction conditions, dicyclohexylborane (HBCy2) and diisopinocampheylborane (HBIpc2) underwent retro-hydroboration to give cyclohexylborane (H2BCy) or isopinocampheylborane (H2BIpc), respectively; these monoalkylboranes act as N2-borylating agents in the presence of a catalytic amount of 1. Under similar conditions, 9-borabicyclononane (9-BBN) slowly adds over the W–N
N unit using various hydroboranes has been examined. In a previous study, we have shown that Piers’ borane (1) reacted with the tungsten dinitrogen complex 2 to afford a boryldiazenido–hydrido–tungsten species. The ease and mildness of this reaction have encouraged us to extend its scope, with the working hypothesis that 1 could potentially catalyse the 1,3-B–H bond addition of other hydroboranes. Under productive reaction conditions, dicyclohexylborane (HBCy2) and diisopinocampheylborane (HBIpc2) underwent retro-hydroboration to give cyclohexylborane (H2BCy) or isopinocampheylborane (H2BIpc), respectively; these monoalkylboranes act as N2-borylating agents in the presence of a catalytic amount of 1. Under similar conditions, 9-borabicyclononane (9-BBN) slowly adds over the W–N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N unit without rearrangement to a monoalkylborane. Catecholborane (HBcat) undergoes the 1,3-B–H bond addition without the need for a catalyst. We were not able to build more than one covalent B–N bond between the terminal N of the N2 ligand and the boron reagent with this methodology.
N unit without rearrangement to a monoalkylborane. Catecholborane (HBcat) undergoes the 1,3-B–H bond addition without the need for a catalyst. We were not able to build more than one covalent B–N bond between the terminal N of the N2 ligand and the boron reagent with this methodology.


 Please wait while we load your content...
Please wait while we load your content...