Bright bluish-green emitting Cu(i) complexes exhibiting efficient thermally activated delayed fluorescence†
Abstract
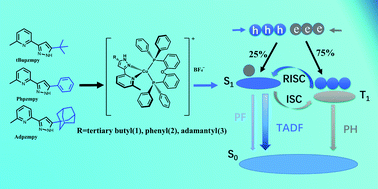
Three strongly emissive Cu(I) complexes [Cu(tBupzmpy)(POP)]BF4(1), [Cu(Phpzmpy)(POP)]BF4(2) and [Cu(Adpzmpy)(POP)]BF4(3) (tBupzmpy = 2-(5-(tert-butyl)-1H-pyrazol-3-yl)-6-methylpyridine, Phpzmpy = 2-methyl-6-(5-phenyl-1H-pyrazol-3-yl)pyridine, Adpzmpy = 2-(5-((3R,5R)-adamantan-1-yl)-1H-pyrazol-3-yl)-6-methylpyridine, and POP = bis[2-(diphenylphosphino)phenyl]ether) were synthesized and characterized. These complexes exhibit bright bluish-green photoluminescence in the solid state with quantum yields of 91% (1), 71% (2) and 77% (3) and lifetimes of 13.4 μs (1), 32.9 μs (2) and 34.1 μs (3) at room temperature. The results of theoretical calculations, coupled with the temperature dependence of the spectroscopic properties and emission decay behaviors, reveal that the title Cu(I) complexes emit efficient thermally activated delayed fluorescence (TADF) from excited states involving metal-to-ligand charge transfer (MLCT) transitions and ligand-to-ligand charge transfer (LLCT) transitions. The emissive-state characteristics and emission properties of the investigated Cu(I) complexes were tuned effectively by changing the steric and electronic structures of the diimine ligands.


 Please wait while we load your content...
Please wait while we load your content...